Npass Pain Scale Is Use Till a Baby Is How Old
Objectives
To provide a procedure for prevention, cess and management of pain in neonates admitted to the Neonatal Unit of measurement. The primary aim is to prevent the potentially damaging effects to the neonatal brain from both the pain experience and the sequelae of medications used to care for hurting and agitation.
Audience
This guideline is applicable to all medical, nursing and midwifery staff caring for neonates and Infants in West of Scotland Neonatal Units.
Pain is defined as an unpleasant sensory and emotional experience associated with actual and potential tissue damage (IASP, 2003).
Neonates cannot verbally communicate their discomfort, however testify suggests that neonates practise feel pain just lack the adaptive mechanisms that modulate painful stimuli in older children. They express their vulnerability to pain and stress through specific behaviours and with physiological and biochemical responses to hurting (Anand et al 2007).
Neonates are frequently exposed to astute, repetitive, and chronic pain within the NICU setting because of procedures, surgeries, and affliction processes. Preterm neonates, especially those <thirty weeks' gestation, are exposed to 10-fifteen painful procedures per twenty-four hours at a time when pain is developmentally unexpected. Pain in the neonatal menstruum is frequently unrecognised and undertreated. In infants born extremely preterm (gestational historic period ≤29 weeks) greater numbers of painful procedures have been associated with poor early neurodevelopment, altered encephalon evolution, delayed postnatal growth and college cortical activation (Beatriz et al. 2015).
It has also been reported that in toddlers built-in very preterm (gestational historic period ≤32 weeks) bio-behavioral pain reactivity-recovery scores have been associated with negative affectivity temperament. Furthermore high numbers of painful experiences in the neonatal period have been associated with a poor quality of cognitive and motor development at ane yr of age and changes in cortical rhythmicity and cortical thickness in children at seven years of age (Beatriz et al. 2015). Few longitudinal studies have examined the impact of neonatal pain in the long-term development of children built-in preterm, however neonatal hurting-related stress is associated with alterations in both early and in subsequently developmental outcomes (Beatriz et al. 2015).
It is therefore recommended that appropriate methods of assessing pain are applied, such as a validated pain cess scale NPASS (Neonatal Pain Agitation and Sedation Calibration) and advisable interventions implemented.
There are a wide range of causes of pain to the neonate. It is important that each procedure/event is assessed and advisable analgesia/support is given.
Summary of Hurting Relieving Interventions for Neonatal Procedures
| Procedure | Interventions | Comments | |
| Non-pharmacological | Pharmacological | ||
| NGT/OGT insertion | Breast milk | ||
| Urinary catheter placement | Breast milk | ||
| Tape removal | Breast milk | ||
| Intramuscular injections Immunisations | Breast milk | Paracetamol | |
| ROP /indirect opthalmoscopy | Breast milk | Local analgesic eye drops | Prior to exam |
| Supra pubic aspiration | Breast milk | ||
| Heel stick | Chest milk | ||
| PICC line placement | Breast milk | Morphine | For intubated patients |
| Lumbar puncture | Breast milk | ||
| Chest drain | Lidocaine | For intubated patients | |
| Intubation | Fentanyl Ketamine | Slow bolus to avoid breast wall rigidity Neonates with hypoplastic left center syndrome/unstable apportionment | |
Starting doses for each medication are every bit per the MCN for West of Scotland Guideline, but dosing and weaning should be individualised
Astute hurting- Consequences of acute pain which may be experienced by the neonate include; astute increases in middle charge per unit, claret pressure, intracranial force per unit area and decreases in oxygen saturations. These changes in cerebral blood flow may exist associated with an increased take a chance of IVH and PVL. It has as well been suggested that acute pain episodes experienced within the neonatal flow may have long-lasting effects on time to come behavioural hurting responses (Taddio et al 1997).
Repetitive pain - Consequences of repetitive or prolonged pain during the neonatal period may result in developmental alterations of the immature nervous system. Preterm infants may accept specific learning deficits, poor adaptive behaviour and decreased pain threshold which tin can change their response to future episodes to painful or non-painful stimuli. Exposure to repeated painful stimuli early in life is known to take short and long-term adverse sequelae. These sequelae include physiologic instability, altered brain development, and abnormal neurodevelopment (Vinall and Grunau 2014).
Chronic hurting- Chronic pain has been described every bit a pathological pain state without apparent biological value that has persisted beyond the normal tissue healing fourth dimension (Jovey 2002) suggesting that the finish of the pain state is not known. Chronic disease atmospheric condition such equally bronchopulmonary dysplasia (BPD) and some surgical conditions can produce chronic repetitive pain and stress on the neonate. However there are no working definitions or validated assessment scales which tin can appraise an extended menstruum of chronic pain in the neonate and is an surface area for future study.
It is important to consider each procedure/upshot and avert or limit where possible.
Limitation or avoidance of peel-breaking or other painful procedures
- Review proposed blood investigations daily and limit claret tests to those necessary for clinical care and management of the baby.
- Avoid multiple heel stick/venepunctures by clustering intendance.
- Consider purposely building in days when no "routine" bloods are done on selected babies.
- Testify suggests that venepuncture is less painful to the neonate than heel stick, therefore venepuncture should be considered for blood tests in neonates who have no venous access bug. Venepuncture attempts should be limited as per Unit of measurement policy (east.chiliad., a maximum of 2-iii attempts per person).
- Suctioning can be painful and should be performed simply when necessary.
- Procedures which may crusade pain or distress to the baby should not be carried out on the same twenty-four hour period e.one thousand. (retinopathy of prematurity exams, immunizations, etc.).
- Care giving activities/procedures should be planned on an individualised ground to allow the baby to fully recover from painful interventions and ensure undisturbed balance.
Limit environmental stressors by reducing dissonance and light levels.
Interventions may include:
- Close incubator doors gently
- Adjust alarms to an advisable level
- Appropriate vox levels at the bedside
- Avoid placing telephones/radios/pages close to incubators
- Utilise incubator covers or drapes to subtract light levels as appropriate for each baby.
a) Cluster nursing intendance and interventions where appropriate and limit treatment of the infant to permit undisturbed residual.
b) Pain and stress may also be alleviated past providing boundaries, swaddling, positioning infants with flexion of extremities, and using pacifiers/encouraging non-nutritive sucking.
c) Parental involvement and interaction should exist actively encouraged and should be an integral part of the infant's care
Interventions may include:
- kangaroo care/skin to skin
- facilitated touch on/gentle massage
- breastfeeding when appropriate
For farther data on Developmental Intendance please refer to the WoS Developmental Care Guideline
Fundamental Line Access
In the first few days of life an umbilical arterial catheter (UAC) / umbilical venous catheter (UVC) may be required for blood sampling, claret pressure monitoring and assistants of intravenous fluids. This may forbid frequent heel stabs for blood sampling and excessive handling. However, the benefits of central admission lines should be carefully balanced with the risks e.k. infection.
Place bodily or potential sources of hurting/irritability.
- These include indwelling tubes or lines, heel-sticks, surgical procedures, suctioning, peritonitis, other infectious processes, fractures, hunger, peripheral and central lines infusions and noxious environment.
- Pain cess is performed with each potentially painful clinical intervention to evaluate the efficacy of behavioural, environmental and pharmacological agents.
- Not-pharmacologic measures are implemented outset if the infant has no identifiable cause for pain.
Suggestions for frequency of pain assessment:
- Invasive tubes or lines: 2-4 hourly
- Receiving scheduled or infusion analgesics and/or sedatives: two-4hrly.
- Analgesic/sedative prn: one hour subsequently dose is given, to assess response.
- Mail service-operative: hourly for 24-48 hours and then four hourly until off medication.
- Handling is initiated based on assessment which includes an objective pain scale.
- Employ pain/agitation scale with all painful procedures.
- After providing hurting management obtain pre-procedure hurting assessment.
- Sedatives practice not provide hurting relief only some may enhance the effects of opioids.
- Sedatives must not be used in identify of analgesics for the management of pain.
- There is no evidence to support that infants can be safely sedated for weeks or months.
- Some sedatives such as benzodiazepines must be used with caution and are not recommended in preterm infants. Potential side effects include myoclonic movements and adverse neurological event.
- Sedatives may withal be considered when ongoing analgesics are necessary.
Assessment of Sedation
- The assessment of sedation levels should exist based on the infant's response to stimuli only when "hands on" care is being given.
- The babe should non be stimulated unnecessarily to appraise sedation.
- Sedation does NOT Demand to be assessed with every pain cess.
- Evidence of sedation without administration of sedatives may indicate neurological depression, sepsis or other pathology.
- Assessment of sedation in infants not receiving pharmacological sedation should be carried out on an as required footing.
- Premature infants who have experienced prolonged periods of untreated pain and stress may become lethargic and "shut downwards" in response.
- Infants who are muscle relaxed cannot be evaluated behaviourally for pain and sedation.
- Medication doses should exist reviewed regularly when paralysis is discontinued.
- Physiological changes such as eye rate and blood pressure level may exist the only indicator of a need for review of medication.
In that location are a plethora of neonatal hurting assessment scales in the literature (Appendix one). Each Neonatal Unit should select a validated pain assessment scale and become experienced in its employ for pain measurement.
Within the West of Scotland MCN the N-PASS (Neonatal Pain Agitation and Sedation Scale) is recommended for use. The N-Pass is a validated pain assessment scale adult in North America for the assessment of pain (page 12) and sedation (page 13) in both term, preterm and surgical neonates utilising multidimensional indicators of neonatal pain. Information technology is crucial that all staff using the N-PASS are trained in use of the scale in order to ensure appropriate pain assessment.
When using the N-PASS pain and sedation are scored SEPARATELY.
It is recommended that hurting levels should be assessed using the North-Laissez passer:
- On admission to the Neonatal Unit
- Minimum of once per shift, frequency thereafter dependent on the babe.
- At every vital sign assessment if receiving regular analgesia/sedation.
More frequent pain cess indications:
- Indwelling tubes or lines which may cause pain, especially with movement (e.g. chest tubes) → at least every two-iv hours
- Receiving analgesics and/or sedatives → at least every two-4 hours
- 30-60 minutes after an analgesic is given for hurting behaviors to appraise response to medication
- Mail-operative → at least every two hours for 24-48 hours, then every 4 hours until off medications
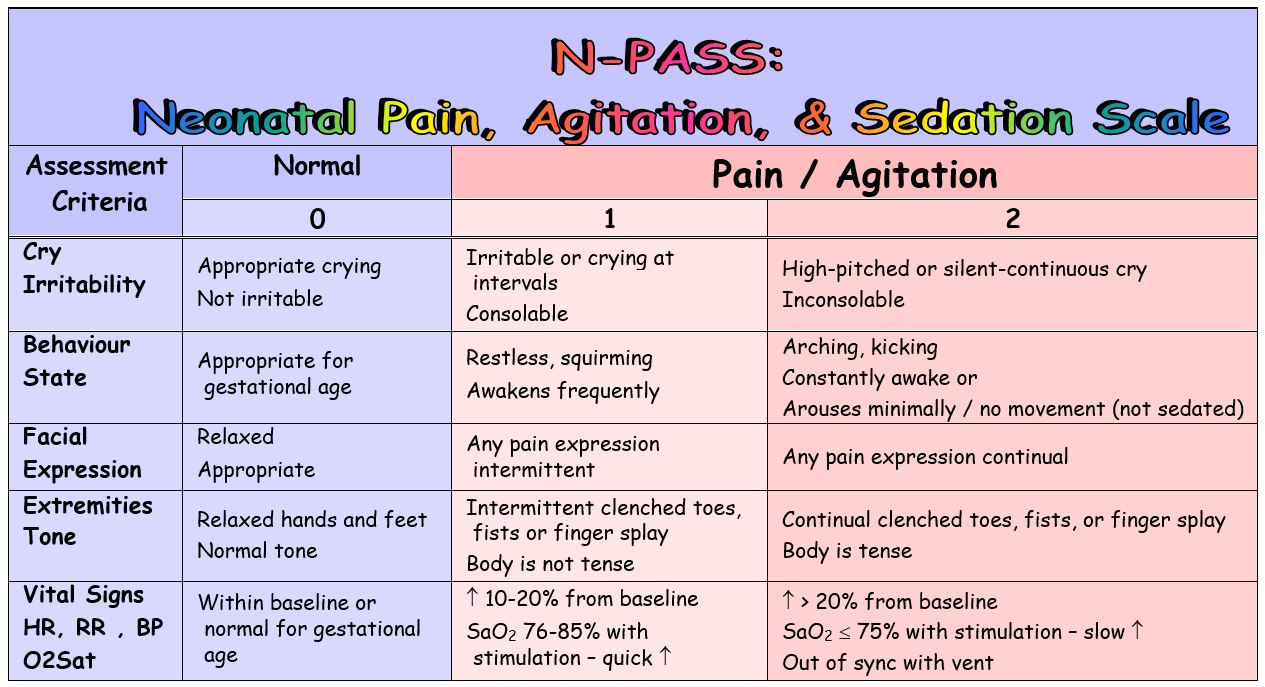
Premature Neonates: + 1 if <30 weeks gestation / corrected historic period
Assessment of Hurting/Agitation
- Hurting assessment is the fifth vital sign – cess for pain should be included in every vital sign assessment
- Pain is scored from 0 → +ii for each behavioral and physiological criteria, then summed
- Points are added to the premature infant'due south hurting score based on their gestational age to recoup for their limited ability to behaviorally or physiologically communicate hurting
- Total pain score is documented every bit a positive number (0 → +10)
- Treatment/interventions are indicated for scores > 3
- Interventions for known hurting/painful stimuli are indicated before the score reaches 3
- The goal of hurting treatment/intervention is a score ≤ 3
- More frequent pain assessment indications:
- Indwelling tubes or lines which may crusade pain, specially with movement (eastward.g. chest tubes) → at least every 2-4 hours
- Receiving analgesics and/or sedatives → at least every 2-4 hours
- xxx-hr later an analgesic is given for pain behaviors to assess response to medication
Post-operative → at to the lowest degree every 2 hours for 24-48 hours, and then every 4 hours until off
Musculus Relaxed
- It is impossible to behaviorally evaluate a paralyzed infant for pain
- Increases in middle rate and blood pressure may be the just indicator of a need for more analgesia
- Analgesics should be administered continuously by drip or around-the-clock dosing
- Higher, more than frequent doses may be required if the infant is post-op, has a breast tube, or other pathology (such as NEC) that would normally cause pain.
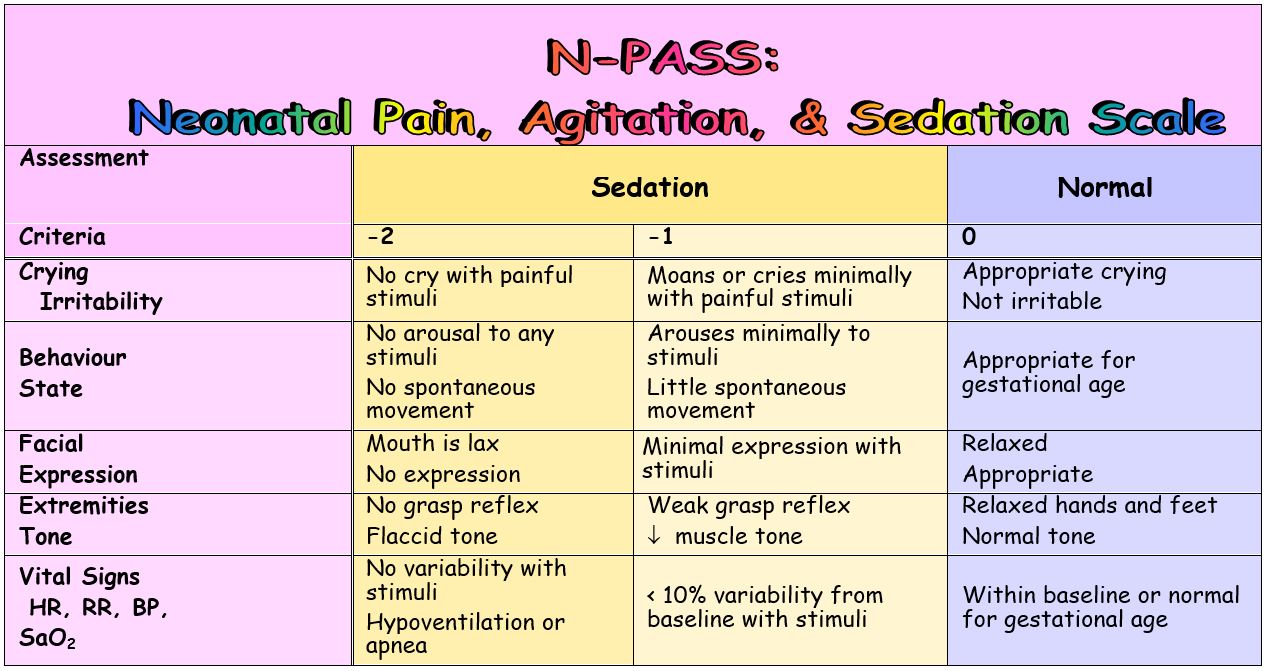
Assessment of Sedation
- Sedation is scored in addition to pain for each behavioral and physiological criteria to assess the infant's response to stimuli
- Sedation does not need to exist assessed/scored with every hurting assessment/score
- Sedation is scored from 0 → -two for each behavioral and physiological criteria, so summed and noted as a negative score (0 → -10)
- A score of 0 is given if the babe's response to stimuli is normal for their gestational age
- Desired levels of sedation vary co-ordinate to the situation
- "Deep sedation" → score of -10 to -five equally goal
- "Light sedation" → score of -5 to –2 as goal
- Deep sedation is not recommended unless an baby is receiving ventilatory support, related to the high potential for apnea and hypoventilation
- A negative score without the administration of opioids/ sedatives may indicate:
- The premature baby'southward response to prolonged or persistent pain/stress
- Neurologic depression, sepsis, or other pathology
Muscle Relaxant
- It is impossible to behaviorally evaluate a muscle relaxed babe for hurting
- Increases in heart charge per unit and blood pressure may be the only indicator of a need for more analgesia
- Analgesics should be administered continuously by drip or around-the-clock dosing
- Higher, more than frequent doses may be required if the infant is post-op, has a chest tube, or other pathology (such as NEC) that would usually crusade pain
- Opioid doses should be increased by ten% every 3-5 days as tolerance will occur without symptoms of inadequate pain relief
The following information relays the assessment criteria used to score the Northward-PASS pain assessment scale.
N-Laissez passer Assessment Criteria
Crying / Irritability
-2 → No response to painful stimuli, e.g.:
- No cry with needle sticks
- No reaction to ETT or nares suctioning
- No response to care giving
-one → Moans, sighs, or cries (audible or silent) minimally to painful stimuli, e.g. needle sticks, ETT or nares suctioning, care giving
0 → Not irritable – appropriate crying
- Cries briefly with normal stimuli
- Easily consoled
- Normal for gestational historic period
+1 → Infant is irritable/crying at intervals – only can be consoled
- If intubated – intermittent silent cry
+2 → Any of the post-obit:
- Cry is high-pitched
- Infant cries inconsolably
- If intubated – silent continuous cry
Behavior / State
-two → Does not arouse or react to any stimuli:
- Eyes continually shut or open
- No spontaneous motility
-1 → Little spontaneous move, arouses briefly and/or minimally to any stimuli:
- Opens eyes briefly
- Reacts to suctioning
- Withdraws to pain
0 → Behavior and land are gestational age appropriate
+1 → Whatever of the following:
- Restless, squirming
- Awakens often/easily with minimal or no stimuli
+2 → Any of the following:
- Boot
- Arching
- Constantly awake
- No move or minimal arousal with stimulation (inappropriate for gestational age or clinical situation, i.e. post-operative)
Facial Expression
-2 → Any of the post-obit:
- Rima oris is lax
- Drooling
- No facial expression at residuum or with stimuli
-ane → Minimal facial expression with stimuli
0 → Face is relaxed at rest but not lax – normal expression with stimuli
+1 → Any hurting face up expression observed intermittently
+ii → Any pain face expression is continual
Extremities / Tone
-two → Any of the following:
- No palmar or planter grasp tin can exist elicited
- Flaccid tone
-1 → Any of the following:
- Weak palmar or planter grasp can be elicited
- Decreased tone
0 → Relaxed hands and feet – normal palmar or sole grasp elicited – appropriate tone for gestational age
+1 → Intermittent (<xxx seconds elapsing) observation of toes and/or hands as clenched or fingers splayed
- Body is not tense
+2 → Any of the following:
- Frequent (≥30 seconds duration) observation of toes and/or easily every bit clenched, or fingers splayed
- Body is tense/stiff
Vital Signs: HR, BP, RR, & O2 Saturations
-ii → Any of the following:
- No variability in vital signs with stimuli
- Hypoventilation
- Apnea
- Ventilated infant – no spontaneous respiratory endeavor
-ane → Vital signs testify picayune variability with stimuli – less than 10% from baseline
0 → Vital signs and/or oxygen saturations are within normal limits with normal variability – or normal for gestational age
+1 → Whatsoever of the following:
- 60 minutes, RR, and/or BP are 10-20% to a higher place baseline
- With care/stimuli infant desaturates minimally to moderately (SaO2 76-85%) and recovers chop-chop (inside two minutes)
+2 → Any of the following:
- Hr, RR, and/or BP are > 20% above baseline
- With care/stimuli baby desaturates severely (SaOtwo < 75%) and recovers slowly (> ii minutes)
- Babe is out of synchrony with the ventilator –fighting the ventilator
1) The best approach to direction of neonatal hurting is prevention.
- Hurting assessment should be ongoing and incorporated into the plan of care for all neonates with potential and bodily pain experiences.
- Limit potentially painful procedures and administer appropriate analgesics when pain is anticipated or prior to procedures.
two) Non-pharmacological Interventions
- Breast milk, offer breast feed, swaddling, kangaroo care, facilitated affect, etc. may assistance alleviate pain and should be used, when advisable, prior to and during painful procedures (such as blood sampling, PIV or PICC placements).
- Parental involvement has been demonstrated to reduce pain and should be allowed when all involved parties are comfortable.
three) Pharmacological Interventions- Oral and Topical
Oral Analgesics
- Oral sucrose
Expressed chest milk or oral sucrose may be used for procedural pain as per the MCN West of Scotland Neonatal Guideline. Oral sucrose is not to be used solely for irritability, or for chronic pain.
- Oral Paracetamol
May exist used for management of hurting in particular post-operative pain relief. Too may be used following vaccinations.
- Oral Opiates
Oral morphine may be given for procedural hurting. Likewise may be used equally a weaning program when extended use of 4 morphine has been necessary.
Topical or Local Anaesthetics
Local anaesthetics may be applied to the skin earlier clinical procedures. The cream should exist practical to no more three areas at the aforementioned time.
In that location are two types of local anaesthetic cream: Ametop Gel and EMLA foam.
EMLA cream is licenced for infants over i year of age and therefore not oft used inside neonatal units.
- Ametop Gel
Ametop Gel contains the anaesthetic tetracaine and is licenced for children over one month of historic period. Information technology is applied 30 to 45 minutes before the procedure and wiped off before the process begins, it should not be left on the skin longer. The site remains numb for iv to half-dozen hours. It increases the size of the claret vessels where it is practical and therefore can cause temporary redness.
Potential Side Effects of the Creams
There tin can be allergic reactions to the creams, causing itching, swelling or bruising where it is practical. Some changes in pare colour may occur where information technology is applied however this is normal.
Local anaesthetics should Not be used on:
- wounds, broken pare or eczema
- the ears, nose , eyes, rima oris or lips
- genitalia or anus
- Lidocaine
Lidocaine may be administered subcutaneously for procedures such every bit breast bleed insertion. Given at least one to two minutes earlier the process.
IV Pharmacological Interventions:
i) Morphine
- Appropriate in neonates without hypotension
- Should unremarkably exist given on a PRN basis in preterm neonates
- May be given pre-emptively in older babies
- Complications include respiratory depression, hypotension, bladder distention/urinary retention, feeding intolerance
- Caution should exist exercised in neonates <26 weeks gestational age or those with pre-existing hypotension
- Opioids for Neonates Receiving Mechanical Ventilation
Mechanical ventilation is potentially a painful intervention for the neonate. Opioids have historically been used within neonatal intensive intendance to ease any pain or discomfort experienced. Still there is insufficient evidence to indorse the routine utilise of opioids for neonates requiring mechanical ventilation. It has been reported that opioids are no improve or worse for ventilated babies (in terms of time to come development, duration on ventilation, death, strokes or hospital stay) than other drugs or placebo. It is recommended that opioids should exist used selectively, when indicated and guided by clinical judgement and indicators of neonatal pain (Bellu et al 2010).
- Weaning from Opioid Treatment
It may exist necessary to instigate a weaning programme for those neonates who have had an extended menses of fourth dimension on opioids (Appendix 2). This should be managed in conjunction with advisable pain assessment.
ii) Fentanyl
- May be used in younger neonates and those with hypotension
- Should usually be given on a PRN ground in preterm neonates
- Complications include respiratory depression, chest wall rigidity (especially if given too quickly), mild hypotension
- Used only in babies who are ventilated due to gamble of breast wall rigidity.
three) Paracetamol
- May be considered for post-operative patients who are nil by mouth
- May exist used in combination with opiates
- Circumspection in hepatic impairment or neonates with unconjugated hyperbilirubinaemia
- For appropriate dose range please refer to the W of Scotland Neonatal IV Drug Monographs.
iv) Sedatives for the treatment of stress/anxiety
Benzodiazepines may be given for associated feet/stress but are non appropriate for the treatment or prevention of pain and not recommended for use in the preterm neonate.
v) Nurse Controlled Analgesia (NCA)
Nurse controlled analgesia (NCA) refers to a modified morphine infusion using technology which permits more flexibility to manage quantum pain than a simple continuous infusion. Information technology is used in limited clinical areas in the W of Scotland generally in the immediate post- operative period. The nurse caring for the patient may press a button to give a bolus dose on the footing of a request for analgesia, hurting severity scoring or in anticipation of pain.
Nurse Controlled Analgesia (NCA) is a technique of morphine administration appropriate for some neonates. NCA is delivered through a specifically designed locked pump which allows for programming of a bolus dose to exist delivered by nursing staff on the press of the NCA push button. Normally the bolus is x – 20 micrograms/kg with a 20-30 minute lockout menstruum. A low rate of background infusion may also be used aslope the bolus, and is ready from 4micrograms/kg/hr upwards.
Although this technique potentially allows for larger doses of opiates to exist administered, experience shows that the same level of groundwork pain relief is accomplished with much reduced total opiate dose. This is considering of the dynamic nature of the administration which allows for doses to be given exactly at the moment the patient is experiencing pain or to pre-empt painful/ deplorable procedures such as endotracheal suction.
At its most effective, when used in tandem with regular pain scoring NCA volition tend to wean itself. For example, equally the patient recovers from the pain of surgery their hurting scores will improve and the NCA will crave fewer presses. Reduction in total opiate load has the potential advantages of reduced respiratory support and more rapid return of GI function postal service-surgery.
Patient Selection
Patients selected for NCA normally have (or are expected to accept) severe astute pain for whom the oral route is not appropriate, such as during the firsthand post-operative period. Prior to setting up the infusion the anaesthetist or acute pain service staff will consider the suitability of NCA for each individual patient and the anticipated effectiveness of NCA for the type of surgery / pain.
Absolute contraindications to NCA are:
- Known allergy to opioids (very rare)
Actress circumspection is advised when using a NCA in patients with sure medical conditions:
- Raised intra-cranial pressure (ICP): Known or suspected raised ICP of any cause represents a relative contraindication to NCA analgesia, although in advisable circumstances its use in areas with high nurse/patient ratios may however be fitting.
- Severe respiratory disease: NCA should be used with caution in these Patients
- Children with renal damage or sensitivity to morphine
- No supplementary opioids should be administered while the patient is receiving NCA
- NCA pumps for children must just be programmed by an anaesthetist, fellow member of the Acute Pain Service or appropriately trained health professional.
- All personnel who care for patients receiving NCA must be trained and competent to practise so.
- All pumps should be kept locked while in apply and a dedicated line should be used. The anti-siphon line should be connected directly to the patients intravenous admission or through a 2 manner non-return connector
- Parents should be advised in a sensitive way that they are non permitted to administer a bolus by pressing the button
- Nursing staff should acquit out advisable hurting assessment using local pain assessment tool and certificate hourly.
half dozen) Neonatal Regional Techniques
Neonates can nowadays a unique challenge in the direction of post-operative pain. Regional techniques are beingness increasingly used to minimize post-operative pain.
Epidural
The most commonly used technique is a single dose administration of local anaesthetic amanuensis into the caudal (epidural) infinite for operations such equally inguinal hernia repair. This provides adequate pain relief during the surgery itself, and for up to iv hours afterwards.
Another commonly used regional technique involves placement of an epidural catheter in the epidural space in theatre for prolonged hurting management. The epidural catheter may take been inserted into the caudal space and advanced to a level in the back that corresponds to the level of surgical incision, or may have been inserted higher up the dorsum at this level. For surgery, where significant pain may be a predicted problem mail-operatively, for example laparotomy, a local anaesthetic infusion pump is attached to the catheter to provide ongoing pain relief. Strict monitoring of the patient and pump infusion rates is in accordance with local Astute Pain Team guidelines and protocols. Epidural infusions would normally run for two to 3 days post operatively. Observed benefits from this technique are potentially earlier extubation, early on return of bowel role, and a reduction in morphine requirement.
More recently single shot spinal anaesthesia, combined with a single shot caudal injection in ex-premature neonates is being performed for surgery below the umbilicus. This is perceived to be beneficial in reducing the risk of post-operative apnoea's in this grouping of patients past avoiding the need for general anaesthesia, and can result in earlier return to feeding. This technique will result in the neonate non existence able to movement their legs for nigh an hour after the local anaesthetic injection. Movement of their legs should be observed to return to normal after this fourth dimension period.
Extrapleural Catheters
Extrapleural catheters are placed by the surgeons in neonates undergoing cardiac or thoracic surgery. Local anaesthetic infusion pumps volition be used in a like way to epidural pumps to provide ongoing pain relief.
| Pain Measure | Age | Pain | Indicator | Psychometric Properties |
| Pain Cess Tool (PAT) (Hodgkinson et al. 1994) | < iii years of age unable to verbalise pain | Prolonged (post-operative) | Sleep design | Content validity |
| Neonatal Pain Agitation and Sedation Calibration (North-PASS) (Hummel et al. 2003) | <28 weeks -Term Corrected for prematurity | Prolonged Mechanical ventilation or postoperative | Behavioural country | Preliminary reliability and validity in progress. |
| Neonatal Infant Pain Calibration (NIPS) (Lawrence et al 1993) | Preterm and term | Procedural | Weep | Content validity |
| Premature Infant Hurting Profile (PIPP) Stevens et al. 1996) | Preterm and term | Procedural | Behavioural state | Content validity |
Four Morphine → Oramorph
- Calculate the total daily IV morphine dose in micrograms
I.eastward. Current dose in mcg/kg/hour X working weight X 24
- Multiply this figure by 2 (for bioavailability) to give total daily oral dose
- Dissever this into six equal doses and prescribe iv hourly in micrograms
- Stop the Four infusion subsequently the 2nd oral dose
Weaning Oramorph
- Wean by 20% of the original dose every 24 hours equally tolerated
- If not tolerated, pace back to previous dose and reattempt the wean 48hours later on
- One time 70% of the dose is weaned, begin extending the dosing interval
- Switch to PRN, max 12 hourly, once ready to wean from twice daily
Anand Chiliad.J, Hall R.W, Desai N, Shephard B, et al. (2004). Effects of morphine analgesia in ventilated preterm neonates: principal outcomes from the NEOPAIN randomised trial. Lancet: 363(9422)1673-82.
Anand KJ, Aranda JV, Berde CB, Buckman Southward, et al. (2006) Summary proceedings from the neonatal pain control group. Pediatrics ;117:S9-S22.
Anand KJ (2001) International Evidence-Based Group for Neonatal P. Consensus statement for the prevention and management of pain in the newborn. Curvation Pediatr Adolesc Med 155:173-80.
Anand One thousand.J.South, Stevens B.J and McGrath P.J (2007) Pain in Neonates and Infants (3rdEdition). London: Elsevier
Barker D.P, Rutter Northward. (1995) Exposure to invasive procedures in neonatal intensive intendance unit of measurement admissions. Arch Dis Child Fetal Neonatal Ed, 72(1):F47-eight.
Barr RG (1998) Reflections on measuring pain in infants: dissociation in responsive systems and "honest signalling". Archive of Diseases in Childhood Fetal and Neonatal Edition 79 (1): 152-156.
Beatriz, V.O., Holsti, L., Linhares, Grand. (2015) Neonatal Pain and Developmental Outcomes in Children Born Preterm: A Systematic Review. Clinical Periodical of Pain. 31(iv) pp355-362
Bellú R, de Waal K, Zanini, R. (2010) Opioids for neonates receiving mechanical ventilation: a systematic review and meta-analysis. Arch Dis Kid Fetal Neonatal Ed; 95(4):F241-51.
Craig KD, Whitfield MF, Grunau RVE, Linton J and Hajistavropoulos Hard disk (1993) Hurting in the preterm neonate: behavioural and physiological indices. Pain 52(three): 287-299
Duhn L and Medves J (2004) A systematic integrative review of baby pain assessment tools. Advances in Neonatal Care four (3): 126-140
Elserafy F, Alsaedi SA, Louwrens J, Bin Sadiq B, et al. (2009) Oral sucrose and a pacifier for pain relief during simple procedures in preterm infants: a randomized controlled trial. Ann Saudi Med, 29(3):184-eight.
Gibbons Southward, Stevens B, Hodnett Eastward, Pinelli J, Ohlsson A and Darlington G (2002) Efficacy and safety of sucrose for procedural pain relief in preterm and term neonates. Nursing Enquiry 51(6): 378-382
Grunau RE and Tu MT (2007) Long- term consequences of pain in human being neonates IN: Anand KJS, Stevens BJ and McGrath PJ (eds) Pain in Neonates and Infants. London: Elsevier 45-55
Hall RW, Huitt TW, Thapa R, Williams DK, Anand KJ, Garcia-Rill E. (2008) Long-term deficits of preterm birth: bear witness for arousal and attentional disturbances. ClinNeurophysiol,19:1281-91.
Hall RW. (2012) Anesthesia and analgesia in the NICU. Semin Perinatol, 39(i):239-54.
Hamers JHP, Abu Saad HH, van den Hout MA, Halfens RJG and Kester ADM (1994) The influence of childrens vocal expression, age, medical diagnosis and information obtained from parents on nurses' pain assessments and decisions regarding interventions. Hurting 65(1): 53-61
Holsti Fifty, Grunau RE. (2010) Considerations for using sucrose to reduce procedural hurting in preterm infants. Pediatrics,125(5):1042-7.
Hudson-Barr DC, Duffey MA and Holditch-Davis D (1998) Pediatric Nurses use of behaviours to brand medication administration decisions in infants recovering from surgery. Enquiry in Nursing Health 21(one): 3-xiii
Hummel P, Puchalski M, Creech SD, Weiss MG. (2008) Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged hurting. J Perinatol, 28(1):55-60.
IASP Job Strength on Taxonomy (2003) IASP Hurting terminology. Available from: http://world wide web.iasp-pain.org/terms-p.html
Jovey RD (2002) Opioids, pain and habit. In: Jovey RD (ed) Managing pain: the Canadian Healthcare Professionals Reference, pp63-77. Rogers Media, Toronto, Canada
McGrath PJ (1996) There is more than to pain measurement in children than "ouch". Canadian Psychology 37 (2): 63-75
Merskey Yard and Bogduk Due north (1994) Classifications of Chronic Hurting: Descriptions of Chronic Hurting Syndromes and Definitions of Pain Terms. Seattle: (IASP) Press
Mokhnach 50, Anderson M, Glorioso R, Loeffler Thou, et al.(2010) NICU procedures are getting sweeter: evolution of a sucrose protocol for neonatal procedural pain. Neonatal Netw 29(5):271-9.
Ng E., Taddio A., Ohissin A. (2017) Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database of Systematic Reviews Event ane Art No CD 002052. DOI: 10. 1002/14651858. CD002052 pub 3
O'Sullivan A, O'Connor M, Brosnahan D, McCreery G, et al. (2010) Sweeten, soother and swaddle for retinopathy of prematurity screening: a randomised placebo controlled trial. Arch Dis Child Fetal Neonatal Ed, 95(6):F419-22
Owens ME and Todt EH (1984) Pain in infancy: neonatal reactions to heel lance. Pain 20 (i): 77-86
Ramsay Yard (2000) Measuring level of sedation in the intensive care unit. Journal of the American Medical Clan 294: 441-442
Stevens BJ and Johnston C (1994) Physiological responses of premature infants of painful stimulus. Nursing Enquiry 43 (4): 226-231
Stevens BJ, Johnson CC and Grunau RVE (1995) Issues of assessment of pain and discomfort in neonates. JOGNN 24 (9):849-854
Stevens BJ, Riddell RRP, Oberlander TE and Gibbins Southward (2007) Assessment of Pain in Neonates and Infants IN: Anand KJS, Stevens BJ and McGrath PJ (eds) Pain in Neonates and Infant. London: Elsevier 67-86
Streiner DL and Norman GR (2006) Health Measurement Scales (threerd Edition) Oxford: Oxford University Press
Taddio A, Katz J, Ilersich A and Koren G (1997) Effect of Circumcision on Hurting Response during Subsequent Routine Vaccination. The Lancet 349:599-603.
Van Dijk Chiliad, Peters J and Bowmeester N (2002) Are postoperative pain instruments useful for specific groups of vulnerable infants? Clinics in Perinatology 29 (3): 469-491
Van Howe RS (1999) Pain relief for neonatal circumcision: serious pattern flaws? Pediatrics 103 (1):196-197
Vinall J and Grunau RE (2014) Impact of repeated procedural pain-related stress in infants born very preterm. Pediatric Research 2014; 75(five):584–587
Wasz-Hockert O, Michelsson K and Lind J (1987) Weep in various diseases. IN: Lester BM, Boukydis CFZ (eds) Baby Crying: Theoretical and Research Perspectives. New York: Plenum Press
Boosted Resources for Cess Tools
North-PASS. Hummel PA et al. Clinical reliability and validity of the N-Laissez passer: neonatal hurting, agitation and sedation calibration with prolonged hurting. J Perinatol 2007; 28: 55-60.
Pain Cess Tool (PAT) Hodgkinson Thou, Bear 1000, Thorn J, Blaricum South. Measuring hurting in neonates: Evaluating an musical instrument and developing a mutual linguistic communication. J Adv Nurs 1994;12(1):17-22.
The Distress Calibration for Ventilated Newborn Infants (DSVNI) Sparshott Chiliad. The development of a clinical distress scale for ventilated newborn infants: identification of pain and distress based on validated behavioural scores. J Neonatal Nurs 1996;2:v- 11.
The Neonatal Infant Hurting Scale (NIPS) Lawrence J, Alcock D, McGrath J, Kay S, et al. The development of a tool to assess neonatal hurting. Neonatal Network, 1993;12(6):59-66.
Source: https://www.clinicalguidelines.scot.nhs.uk/nhsggc-paediatric-clinical-guidelines/nhsggc-guidelines/neonatology/neonatal-pain-guideline/
0 Response to "Npass Pain Scale Is Use Till a Baby Is How Old"
Enviar um comentário